2-DG effective against all variants of corona virus
⏺️ Claims made in the study on DRDO’s drug 2-DG
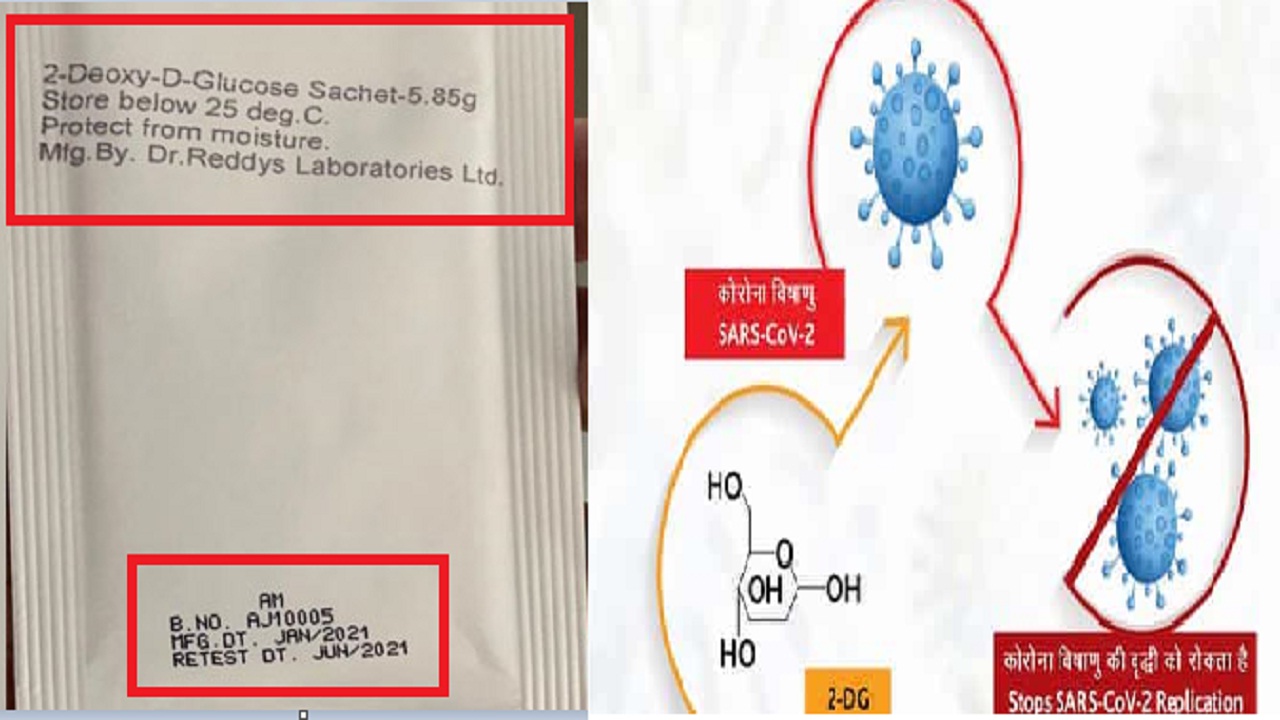
A new study has claimed that the novel corona virus drug 2-deoxy-D-glucose (2-DG), developed by the Defense Research and Development Organization (DRDO), is effective against all forms of COVID-19 and even This drug also reduces the multiplication of SARS-CoV-2 virus.
Preliminary studies have also shown that the anti-COVID drug of DRDO reduces the infection-induced cytopathic effect (CPE) in cells and prevents their elimination. It is worth mentioning that the 2-DG drug was launched by Defense Minister Rajnath Singh and Union Health Minister Dr. Harsh Vardhan on May 17 at DRDO Headquarters. Releasing the first batch of DRDO’s anti-Covid drug, the central government claimed that this drug has the ability to reduce the average recovery time of a patient by two and a half days and oxygen demand by 40 percent. It was granted authorization by the Drug Controller General of India (DCGI) on June 1 for emergency use as an adjunctive therapy for moderate to severe coronavirus patients.
New study review
This new study, published last June 15, has not yet been reviewed. This study report has been prepared by Anant Narayan Bhatt, Abhishek Kumar, Yogesh Rai, Dhivia Vedagiri and others. In the study, DRDO’s anti-COVID drug 2-DG was used to target and inhibit metabolic reprogramming induced by COVID-19 infection in patients with the disease. The results showed that COVID-19 infection causes high influx of glucose into the body and glycolysis in cells, resulting in high accumulation of fluorescent glucose/2-DG analogs and 2-NBDG in a selective manner.
Cautionary Advice
It is noteworthy that doctors suggest some precautions regarding the use of DRDO’s anti-Covid drug 2-DG. The effect of this anti-viral drug on patients suffering from diseases like uncontrolled diabetes, severe cardiac problems and even kidney failure, ARDS etc. is not studied yet. Further, DRDO recommends that 2-DG be prescribed as early as possible in the treatment of moderate to severe COVID-19 patients for a maximum period of 10 days.






